An Allele That Can Mask Another Allele Is Said to Be
Epistasis describes how gene interactions tin impact phenotypes. Did you know that genes can mask each other's presence or combine to produce an entirely new trait?
In his dihybrid crosses with pea plants, Gregor Mendel simultaneously examined two different genes that controlled two different traits. For case, in ane serial of experiments, Mendel began by crossing a plant that was homozygous for both circular seed shape and xanthous seed color (RRYY) with another institute that was homozygous for both wrinkled seed shape and green seed color (rryy). So, when Mendel crossed two of the Fone progeny plants with each other (RrYy × RrYy), he obtained an F2 generation with a phenotypic ratio of 9:iii:3:1, as summarized in Table 1.
Table 1: Phenotypes and Genotypes in Mendel's F2 Generation
| Proportion | Genotype | Phenotype |
| 9/16 | R_Y_ | Circular, yellow |
| 3/16 | R_yy | Round, green |
| iii/sixteen | rrY_ | Wrinkled, yellow |
| 1/16 | rryy | Wrinkled, light-green |
In this dihybrid cross, each factor locus had an independent effect on a single phenotype. Thus, the R and r alleles affected only the shape of the seed and had no influence on seed color, while the Y and y alleles affected only seed colour and had no influence on seed shape. In this case, there were two split genes that coded for ii separate characteristics.
But what happens when two dissimilar loci touch on the aforementioned characteristic? For instance, what if both of the loci in Mendel'southward experiment affected seed color? When 2 genes are involved in the issue of ane characteristic, a dihybrid cross involving these genes can produce a phenotypic ratio very different from 9:3:iii:1. Under these circumstances, there are more than two gene products affecting the same phenotype, and these products may have complex hierarchical relationships. Any fourth dimension two different genes contribute to a unmarried phenotype and their furnishings are non merely condiment, those genes are said to be epistatic.
Although some researchers have attempted to categorize all digenic (2-cistron) epistatic interactions with specific names, those classification schemes are seldom used today. One reason that they have fallen out of favor is that terms such as "dominant" and "recessive" are best used to draw the effects of alleles of unmarried genes. Furthermore, epitasis is not restricted to the interactions of just 2 genes. Rather, epistasis occurs in all of the following scenarios:
- Whenever two or more loci collaborate to create new phenotypes
- Whenever an allele at one locus masks the effects of alleles at one or more other loci
- Whenever an allele at one locus modifies the effects of alleles at one or more other loci
Epistasis is an interaction at the phenotypic level of organization. The genes that are involved in a specific epistatic interaction may still testify contained array at the genotypic level. In such cases, all the same, the phenotypic ratios may appear to deviate from those expected with independent assortment.
Epistatic Relationships Involving Two Genes
Equally previously mentioned, scientists take performed numerous studies in an attempt to improve understand and classify digenic epistatic relationships. Some of the most famous examples of research in which the interaction between ii genes was found to produce a novel phenotype are examined in the following sections.
Combs in Chickens

Figure i: Rooster displaying a unmarried comb.
Eric Isselee/Shutterstock. All rights reserved.
In the showtime decade of the twentieth century, British geneticists William Bateson and R. C. Punnett conducted enquiry showing that the shape of the comb in chickens was caused by the interaction between ii dissimilar genes. Bateson and Punnett were aware of the fact that different varieties of chickens possess distinctive combs. For instance, Wyandottes take a "rose" comb, Brahmas have a "pea" comb, and Leghorns have a "single" comb. When Bateson and Punnett crossed a Wynadotte chicken with a Brahma craven, all of the F1 progeny had a new blazon of comb, which the duo termed a "walnut" comb. In this case, neither the rose comb of the Wyandotte nor the pea rummage of the Brahma appeared to exist dominant, because the Fi offspring had their own unique phenotype. Moreover, when two of these Fone progeny were crossed with each other, some of the members of the resulting Ftwo generation had walnut combs, some had rose combs, some had pea combs, and some had a single comb, similar that seen in Leghorns (Figure 1). Because the four comb shapes appeared in a 9:iii:3:1 ratio (i.e., nine walnut chickens per every 3 rose chickens per every three pea chickens per every one single-comb craven), information technology seemed that two different genes must play a role in rummage shape.
Through continued research, Bateson and Punnett deduced that Wyandotte (rose-combed) chickens must take the genotype RRpp, while Brahma chickens must have the genotype rrPP. A cross between a Wyandotte and a Brahma would yield offspring that all had the RrPp genotype, which manifested as the walnut-rummage phenotype. Indeed, any chicken with at least one rose-rummage allele (R) and one pea-comb allele (P) would have a walnut rummage. Thus, when two Fane walnut chickens were crossed, the resulting Ftwo generation would yield rose-comb chickens (R_pp), pea-comb chickens (rrP_), and walnut-comb chickens (R_P_), as well as chickens with a new, fourth phenotype—the unmarried-comb phenotype. Based on the procedure of emptying, it could be assumed that these unmarried-comb chickens had the rrpp genotype (Bateson & Punnett, 1905; 1906; 1908).
Flower Color in Peas

Bateson and Punnett next performed a gear up of experiments in peas that besides showed gene-gene interaction. The duo opted to use peas because information technology is relatively easy to perform hybrid crosses with these plants, and they chose to focus on the feature of flower color.
Bateson and Punnett began by crossing two varieties of pea, each of which was pure-convenance for white flowers. This cross yielded an F1 generation in which all progeny had purple flowers. Next, two F1 plants were crossed to create the F2 generation. In this generation, Bateson and Punnett counted a total of 382 purple-flowered plants and 269 white-flowered plants. The ratio of purple flowers to white flowers was thus 9.4:6.6, or approximately ix:7.
What could explain this variation from Mendelian ratios? Bateson fix out to answer this question in a 1909 study, in which he first proposed what he called the ability of one "allelomorphic pair" (pair of gene alleles) to mask the affects of the alleles for another factor. To rephrase this in terms of Bateson and Punnett's pea experiment, it seemed that 2 recessive alleles at one flower locus could mask the effects of the alleles at the other flower locus. Let's designate the get-go locus as the C locus, and the second as the P locus. If Bateson'due south theory held true, it meant that any flower with the cc genotype would be white, no matter what alleles were present at its P locus. Similarly, any flower with the pp genotype would also exist white, no affair what alleles were present at its C locus. Bateson later used the word "epistasis," which translates as "continuing upon," to define the masking action of 1 factor by another. (Since then, scientists have come to understand that genes can interact in more ways than just masking.)
Many years after Bateson first described this 9:seven phenotypic ratio in pea plants, researchers were finally able to determine the two genes responsible for information technology (Dooner et al., 1991). These genes control flower color by controlling pea found biochemistry, in particular that related to paint compounds called anthocyanins. In peas, there is a 2-step chemical reaction that forms anthocyanins; gene C is responsible for the starting time step, and factor P is responsible for the second (Figure 2). If either pace is nonfunctional, then no purple pigment is produced, and the affected pea institute bears simply white flowers. The dominant C and P alleles code for functional steps in anthocyanin production, whereas the recessive c and p alleles code for nonfunctional steps. Thus, if two recessive alleles occur for either gene, white flowers will event.
Table 2 shows in particular how the 9:7 ratio is a modification of phenotypic but not genotypic Mendelian ratios. Annotation that the C and P genes independently assort, and remember that the presence of a recessive genotype at one locus (i.eastward., cc or pp) masks the effects of the alleles at the other locus. Annotation besides that there are nine combinations of alleles in the F1 generation that characteristic at least ane dominant C and one dominant P allele, which would yield a purple flower phenotype (indicated within the table past purple shading). Conversely, there are seven combinations that result in either a cc or a pp, which would yield the white flower phenotype-hence, the 9:7 ratio of imperial to white flowers.
Table 2: Results of the Cross Between Two Pea Plants with Genotype CcPp
| Female Gametes | ||||||
| CP | Cp | cP | cp | |||
| Male Gametes | CP | CCPP | CCPp | CcPP | CcPp | |
| Cp | CCPp | CCpp | CcPp | Ccpp | ||
| cP | CcPP | CcPp | ccPP | ccPp | ||
| cp | CcPp | Ccpp | ccPp | ccpp | ||
Primula Petal Colour

Effigy 3:
Malvidin production.
Production of the petal pigment malvidin is controlled by one cistron, but its synthesis can be suppressed by another gene at a dissimilar locus.
Later researchers discovered that flower petal color can also be controlled by dominant epistatic forces. For instance, in the Primula plant, the pigment malvidin creates blue-colored flowers. Synthesis of malvidin is controlled by gene K, even so production of this pigment can exist suppressed by gene D, which is institute at completely different locus (Figure 3). In this instance, the D allele is ascendant to the K allele, so plants with the genotype KkDd will not produce malvidin because of the presence of the D allele.
So, if two plants with genotype KkDd are crossed with each other, what is the ratio of blue offspring to nonblue offspring? The results of such a cantankerous are detailed in Tabular array iii.
Table 3: Results of the Cross Between Two Primula Plants with Genotype KdDd
| Female Gametes | ||||||
| KD | Kd | kD | kd | |||
| Male Gametes | KD | KKDD | KKDd | KkDD | KkDd | |
| Kd | KKDd | KKdd | KkDd | Kkdd | ||
| kD | KkDD | KkDd | kkDD | kkDd | ||
| kd | KkDd | Kkdd | kkDd | kkdd | ||
In Tabular array 3, all of the shaded boxes incorporate genotypes that indicate an absence of malvidin. In detail, the yellow boxes represent genotypes that feature at least i D allele, and the presence of the D allele suppresses the production of malvidin. Meanwhile, the light turquoise box represents a genotype with no malvidin suppression (dd), only too no malvidin production (kk). On the other hand, the three unshaded/white boxes are the only genotypes that allow the production of malvidin, which means that plants with these phenotypes bear bluish flowers. The phenotypic ratio is therefore xiii:iii. This type of epistasis is sometimes called dominant suppression, because the deviation from ix:3:iii:ane is acquired by a single allele that produces a dominant phenotype, and the action of this allele is to suppress the expression of some other gene.
Wheat Kernel Color
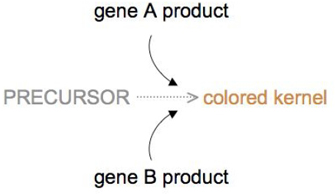
Genes don't always take to deed in opposition to each other for an interaction to be epistatic, however. Sometimes, ii genes that each have the aforementioned role in protein production can substitute for each other.
The mechanism past which wheat kernel color is adamant is an example of indistinguishable gene action. In wheat, kernel colour is dependent upon a biochemical reaction that converts a precursor substance into a pigment, and this reaction tin can be performed with the product of either gene A or gene B (Figure 4). Thus, having either an A allele or a B allele produces color in the kernel, merely a lack of either allele will produce a white kernel that is devoid of colour.
Table 4 depicts the results of a dihybrid cross between two plants with genotype AaBb. Notation that among the offspring of this cross, the phenotype is about uniform.
Table 4: Results of the Cross Between Two Wheat Plants with Genotype AaBb
| Female Gametes | ||||||
| AB | Ab | aB | ab | |||
| Male person Gametes | AB | AABB | AABb | AaBB | AaBb | |
| Ab | AABb | AAbb | AaBb | Aabb | ||
| aB | AaBB | AaBb | aaBB | aaBb | ||
| ab | AaBb | Aabb | aaBb | aabb | ||
In this cross, whenever a ascendant allele is present at either locus, the biochemical conversion occurs, and a colored kernel results. Thus, only the double homozygous recessive genotype produces a phenotype with no color, and the resulting phenotypic ratio of color to noncolor is fifteen:ane.
In the malvidin example in Table 3, gene D is epistatic to gene Chiliad because ane allelic pair masks the expression of an allele at the second locus. In the instance of indistinguishable cistron activity, equally in Table 4, the outcome is less variable, but it is all the same derived from multiple factor interactions. Here, if wheat kernel color is controlled by genes A and B, so A is epistatic to allele B or allele b, and B is epistatic to allele A or allele a.
Coat Color in Horses
Yet another type of epistasis occurs when ane gene interacts with another to modify—simply not mask—a phenotype. For example, in horses, the extension gene determines whether an beast's coat color will be ruby-red or black; hither, the dominant allele E produces black pigment in the coat, while the recessive allele e produces red pigment. All horses with genotype ee are therefore red, nevertheless there are many unlike types of cherry-red horses. These differences exist because of the action of epistatic modifier genes.
One such modifier gene is called cream dilution. The cream dilution factor has two alleles: CCr and C. The CCr allele is semidominant; it dilutes red to yellow in the heterozygous country and red to pale cream in the homozygous state. On the other hand, the C allele has no diluting effect on coat color. Thus, horses with genotype eeCC are anecdote colored, and they have reddish-brown coats, tails, and manes. In contrast, horses with one copy of the CCR allele (genotype eeCCCR ) are palomino (i.e., they accept a gold coat with a white mane and tail), while horses with two copies of the CCR allele (genotype ee CCRCCR ) are cremello (i.e., basically white or cream colored).
Other Types of Epistatic Interactions
Today, scientists know that Mendel's predictions about inheritance depended on the genes he chose to study. Specifically, Mendel carefully selected seven unlinked genes that affected seven different traits. However, unlike the phenotypes that Mendel considered, the bulk of phenotypes are afflicted by more than than one gene. Indeed, most of the characteristics of organisms are much more complex than the characteristics that Mendel studied, and epistasis is ane source of this complication. Epistasis can occur in a variety of different ways and issue in a multifariousness of unlike phenotypic ratios, as illustrated in Table 4. Beyond epistasis, gene-environment interactions further increase the diverseness of phenotypes nosotros meet around us each twenty-four hour period.
Table 4: Examples of Digenic Epistatic Ratios
| Ratio | Description | Name(s) of Relationship (Used past Some Authors) |
| 9:3:iii:1 | Consummate authorisation at both gene pairs; new phenotypes result from interaction between dominant alleles, too as from interaction betwixt both homozygous recessives | Not named because the ratio looks similar independent array |
| nine:4:3 | Complete dominance at both gene pairs; nevertheless, when one factor is homozygous recessive, it hides the phenotype of the other gene | Recessive epistasis |
| ix:7 | Complete say-so at both gene pairs; nonetheless, when either gene is homozygous recessive, it hides the outcome of the other cistron | Duplicate recessive epistasis |
| 12:three:1 | Complete dominance at both cistron pairs; however, when one gene is dominant, information technology hides the phenotype of the other gene | Dominant epistasis |
| xv:ane | Consummate say-so at both factor pairs; yet, when either gene is ascendant, it hides the effects of the other gene | Duplicate dominant epistasis |
| 13:3 | Complete authorisation at both gene pairs; notwithstanding, when either gene is dominant, it hides the effects of the other gene | Dominant and recessive epistasis |
| ix:six:one | Complete dominance at both gene pairs; however, when either gene is ascendant, it hides the effects of the other cistron | Duplicate interaction |
| vii:6:3 | Complete say-so at one gene pair and fractional dominance at the other; when homozygous recessive, the first gene is epistatic to the second gene | No proper noun |
| 3:6:3:4 | Complete authorisation at one factor pair and fractional dominance at the other; when homozygous recessive, either cistron hides the effects of the other gene; when both genes are homozygous recessive, the second gene hides the effects of the first | No proper name |
| 11:5 | Complete dominance for both gene pairs only if both kinds of dominant alleles are present; otherwise, the recessive phenotype appears | No name |
References and Recommended Reading
Bateson, W. Mendel's Principles of Heredity (Cambridge, Cambridge University Printing, 1909)
Bateson, West., et al. Experimental studies in the physiology of heredity. Reports to the Development Committee of the Royal Social club 2, 1–154 (1904)
---. Experimental studies in the physiology of heredity. Reports to the Evolution Committee of the Purple Society 3, ane–53 (1906)
---. Experimental studies in the physiology of heredity. Reports to the Evolution Committee of the Royal Gild 4, 1–60 (1908)
Cordell, H. Epistasis: What information technology means, what it doesn't mean, and statistical methods to detect information technology in humans. Human Molecular Genetics 11, 2463–2468 (2002)
Dooner, H. 1000., Robbins, T. P., & Jorgensen, R. A. Genetic and developmental command of anthocyanin biosynthesis. Annual Review of Genetics 25, 173–199 (1991)
Source: http://www.nature.com/scitable/topicpage/epistasis-gene-interaction-and-phenotype-effects-460
0 Response to "An Allele That Can Mask Another Allele Is Said to Be"
Post a Comment